Healthcare technologies have been emerging with the latest advancements in tech in the new era. However, software is the key to creating and operating medical devices. This has been a huge transformation in the healthcare sector which has helped in diagnosing and treatment or monitoring tools immensely.
In short, whether medical devices are operated independently or are linked with the current system, their functionality is controlled by the medical device software (MDS), an essential component.
It is common knowledge that the integration of medical equipment and technology has revolutionized the development and application of healthcare solutions. The software has outperformed conventional components in medical devices by being easily integrated. More dynamic and flexible solutions have been produced as a result of this integration, encouraging creative and patient-centered methods in the medical field.
You will learn everything there is to know about medical device software development from this blog if you want to build a medical tech startup. It will go through the essential factors and the general procedure for developing reliable and legal software for medical devices. So let’s get right to the details without further ado.
5 Different Types of Software Used in Healthcare Industry
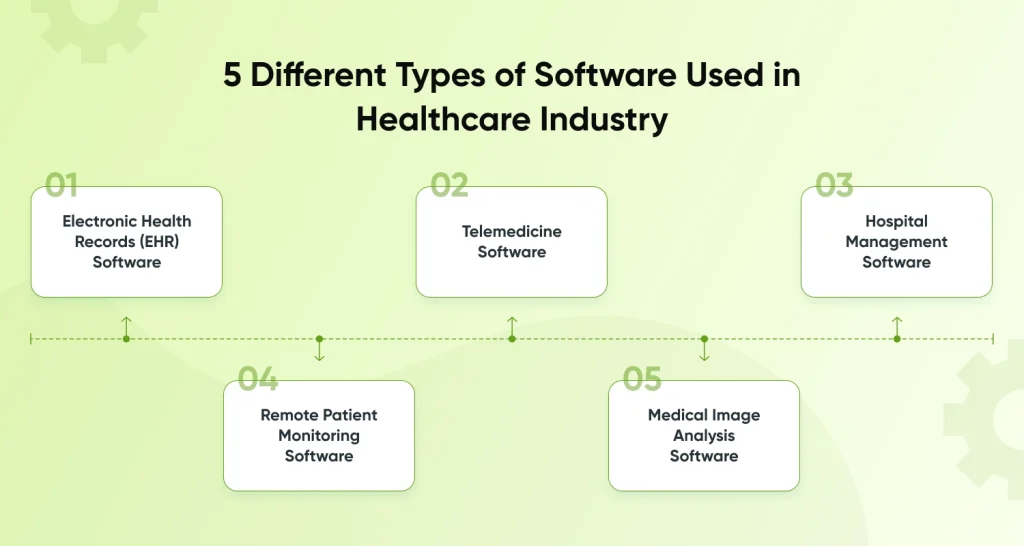
Electronic Health Records (EHR) Software
This well-known and incredibly helpful medical software effectively saves and manages a patient’s whole medical history, including the prescriptions they have taken. It is equivalent to a healthcare CRM designed for the healthcare industry. their diagnosis, the outcomes of their testing, their past and present medical interventions, and so forth.
The main goal of EHR software development for healthcare is to lessen the workload of hospitals and other healthcare institutions by doing away with the necessity for human labor and patient data administration.
Telemedicine Software
While internet consulting was necessary before the pandemic, it is now necessary. The telemedicine software development, which is now necessary for everyone in a period of little to no interaction, is an ideal answer for people living in remote areas or who have disabilities.
A doctor and patient can connect securely, receive answers to questions, schedule a consultation, track their treatment online, or perform a fast examination with the help of telemedicine software.
Hospital Management Software
The goal of hospital management software is to streamline all aspects of a hospital or clinic’s daily operations, from creating bills to arranging visitors. This kind of system can be integrated with software for electronic health records to manage patient data, insurance information, payment processing, and other tasks.
Automating as many administrative tasks as feasible is its primary objective. However, hospital administration software improves operational efficiency.
Remote Patient Monitoring Software
RPM- this monitoring software is to diagnose digitally a patient’s health. RPM tools collect patient health data and send it to a medical practitioner so they can review it and take appropriate action.
Medical Image Analysis Software
Any program that can analyze data from medical photographs and aid in diagnosis, compare images of the same patient over time to assess how the disease is progressing, and provide prognosis is considered medical image analysis software.
A medical web application development can independently detect clinical irregularities in medical images, such as CT, MRI, echocardiography, thermography, and much more, the analytical capabilities of medical imaging software are being upgraded with technology.
Factors to Take Into Account When Developing Software for Medical Devices
The creation of software for medical devices comprises several essential components that need careful consideration and deliberate planning. Businesses can develop software that is secure, efficient, and complies with regulations by carefully taking into account each of these aspects.
Consider Regulation Compliance
Set by the FDA, it is important to follow strict compliance. Adherence to these criteria ensures that medical device software is safe, effective, and of high quality. Regulatory compliance is also essential for meeting standards for market approval and navigating the many legal and quality requirements that guarantee patient safety.
Data Privacy and Security
Encryption and access limits are important to be kept in a secure place for data safety. To build relations and trust between the patient and the healthcare systems, data protection regulations such as HIPAA should be implemented to secure the confidential data of the patient.
User Experience and Usability
You need to develop medical device software that is user-friendly and also serves simple to the healthcare providers. A well-designed and created software will reduce errors and boost productivity. It will be easy for the users and the doctors to communicate.
Communication Between Medical Systems
It is essential to develop software that integrates and communicates with other devices in the healthcare ecosystem. Interoperability is a function that helps seamless data sharing to improve patient outcomes and streamline the delivery of healthcare services.
Risk Assessment
Thorough risk analysis procedures are required to identify potential hazards and lower software-related risks. This incorporates comprehensive risk assessment, management, and mitigation strategies to guarantee patient safety and minimize potential harm.
Cooperation with Stakeholders
It is important to involve stakeholders, including patients, healthcare providers, and regulatory agencies, to ensure that this software will surely solve issues and restore the gaps.This helps to increase its applicability and usefulness in real healthcare settings. This collaboration also guarantees that the program conforms to user and regulatory standards.
Medical Device App Development [7 Easy Steps to Follow]
To guarantee compliance, accuracy, and operational excellence, there are certain crucial procedures to take while developing custom software for medical devices by medical software engineering. Below, let’s examine them in more detail:
Recognize Your Needs Regarding Regulations
Before beginning the medical device software development process, a complete understanding of the intricate regulatory standards, such as those established by the FDA, HIPAA, and other local regulatory bodies, is required.
To involve and inculcate these laws is essential to secure the data and confidential patient data safety. Understanding these standards will influence the scope, features, and safety procedures of the software across the whole life cycle of developing medical devices.
Clarifying User Requirements and Conducting Market Research
To create medical device apps that are effective and easy to use, thorough market research is required. We can obtain a thorough grasp of the state of healthcare, market trends, and user requirements thanks to this study. Through active collaboration with healthcare providers, patients, and other relevant parties, we can pinpoint precise user needs and preferences.
Determining the features, functionality, and design of the software requires these insights. We can guarantee that the final product is more efficient and user-friendly by customizing it to match the unique needs of the users.
Find the Best Development Company
Working with a bespoke medical software development company that has a solid track record and a tonne of experience creating medical software systems is essential. It is also essential that they have a track record of upholding regulatory obligations.
In addition to helping with software development, a reputable medical software development firm like CMARIX can offer insightful advice and helpful insights. Their knowledge will be essential for guaranteeing compliance, producing reliable, excellent software, and providing creative solutions.
Organizing Phase with the Outsourcing Partner Hired
It’s now time to work on project planning with the medical device software development business of your choice. It entails organizing the approach, setting standards, and defining the project’s parameters.
At this critical stage of the software development process for medical devices, the foundation is laid, goals are outlined, and the development process is coordinated with exact dates and targets.
Development and Integration
Following the completion of the required preparations, the software development process for medical devices gets underway. It is crucial to prioritize accuracy, quality, and regulatory compliance at this stage. To enable data sharing between various systems, the software must also seamlessly connect with the healthcare environment.
Implementation and Monitoring
The software will be integrated into the healthcare environment after it is finished. Careful monitoring and performance evaluation of the program are essential throughout this stage of medical device software development. To ensure that the software operates effectively and efficiently, it is essential to continuously monitor any problems or areas that require improvement during the medical device app development process.
Assistance and Maintenance
Maintenance, support, and post-implementation are essential to guarantee the software’s dependability, security, and best possible performance in the medical setting. In this stage of the software development life cycle for medical devices, problems are resolved, updates or patches are applied, and the software is kept current and compliant. Medical Device Software is guaranteed to remain efficient through ongoing support and maintenance.
How Can You Choose the Best Software Company for a Medical Device App Development Project?
If you want to build healthcare scheduling software systems and choose from the best medical device software development companies for your medical device project, there are several things to take into account. Let’s take a quick look at them below:
Total Experience and Proficiency
Analyze the company’s knowledge of healthcare laws and its expertise in creating medical software. This guarantees their capacity to provide solutions that are both compliant and efficient.
References and Portfolio
To make sure they have successfully delivered solutions similar to yours, check out their previous projects. Review case studies and advice from other well-known experts to determine their level of expertise.
Proficiency in Skills
Analyze the team’s skills and knowledge. Make sure their professionals are knowledgeable in medical software specifically, including AI, IoT, and regulatory requirements. This will enable you to determine whether they possess a thorough understanding of the healthcare technology needs that are essential to your project.
Adherence to Regulations
To ensure that the software complies with legal requirements, make sure the organization is knowledgeable about healthcare regulations, compliances, and medical device software development standards including HIPAA, FDA, and GDPR.
Post-deployment Assistance
Check for regular updates, maintenance, and post-deployment support.This guarantees a sustained partnership while providing practical and adaptable solutions in the constantly changing healthcare environment.
Conclusion
Medical device software became a driving force behind advancement, helping healthcare service providers as well as enhancing patient care. Nonetheless, creating software for the medical field calls for both specialized knowledge and proficiency with both software and technology.
For over a decade, CMARIX- a healthcare software development company has been crafting smooth and engaging software solutions for businesses across all industries. As a leading software development company, we’ve demonstrated our ability to deliver creative, client-centered healthcare solutions.
Our medical software developers can guarantee that your software solution meets your objectives because they are experienced with every step of the development of medical equipment. Moreover, our experts enhance the functionality of your medical device software by integrating user-friendly cloud development services and IoT solutions. If you have an innovative concept for medical devices that would shape a healthy future, feel free to contact us.
Frequently Asked Questions
What Types of Medical Devices Require Software Development?
Medical device software development plays a crucial role in the healthcare industry today. It is utilized in many different medical devices, such as imaging systems, surgical robots, insulin pumps, and pacemakers. MDSW can contribute to raising the standard, efficacy, and safety of healthcare.
What Are the Key Regulations for Medical Device Software?
The FDA and manufacturers can recognize and keep track of major adverse occurrences involving medical devices thanks to the MDR requirement. Medical device ISO standards come in a variety of forms. The standards that are most frequently used are 13485, 14971, 10993, and 62304. Speak with a consultant with knowledge of medical device standards if you have any questions about how these standards apply to your device or how to comply with the requirements.
What Is the Tech Stack Used in Healthcare Software Programs?
The tech stack used to develop software for medical devices usually includes several tools and programming languages, including:
Compilers and IDEs: Typical coding integrated development environments.
Low-level programming languages for embedded systems include C, C++, Python, MicroPython, and Java.
Testing Tools: Devices and software for validation and quality control.
Cloud development platforms: Azure, AWS, Google Cloud, and Digital Ocean for managing and storing data.
Front-end development frameworks include Core JavaScript for user interface development, Angular, React, Vue, and Node.js.


![A Complete Guide on Medical Device Software Development [2025]](https://blog.cdn.cmarix.com/blog/wp-content/themes/cmarixinternal/public/images/no-image.png)




